Gelucire 5013 Gattefosse
Agente para enmascarar sabor.

Gelucire 5013 gattefosse. People make our name. Mulations, Gelucire® 50/13—Controlled release and increased bio- availability , Gattefossé PF , first ed., April 99, 3000 ex, 1999. Application Water dispersible surfactant, Solubilizer, Bioavailability.
It has similar surfactive properties to Gelucire® 44/14. The results revealed that the moderate amount of Gelucire 43/01 and ethyl cellulose. Peceol ™ Labrafac ™ lipophile WL 1349;.
Gelucire® 50/13 was a gift by Gattefossè (Milan, Italy). Solid state characterization, kinetic solubility, powder dissolution, stability, and pharmacokinetics were analyzed in rats. CA1 CAA CA CA1 CA A1 C A1 CA A1 CA A CA A CA A CA A C A CA A CA A1 C A1 CA A1 Authority CA Canada Prior art keywords isotretinoin pharmaceutical composition contains group consisting mixtures Prior art date Legal status (The legal status is an assumption.
Development of Solid SEDDS, III:. One excipient that has stimulated interest in lipid-based solid dispersion formulations is Gelucire 44/14 (Gattefosse Corp., St. Legal notices - Terms and condition © Gattefossé People make our name.
Single excipient formulation system:. SAXS analysis of the untreated sample shows a lamellar phase of1Å(figure2).Nosignaloftheacylglycerol fraction was detected. USB2 US11/2,363 USA USB2 US B2 US B2 US B2 US A US A US A US B2 US B2 US B2 Authority US United States Prior art keywords method isotretinoin composition group consisting selected Prior art date Legal status (The legal status is an assumption and is not a legal conclusion.
As discussed previously, even though most Gelucires have T m > 37°C, (the most frequently used for SLN/NLC formulation up to now is Gelucire 50/13), their T m after processing to colloidal dimensions is expected to be decreased, leading to only partially crystallized lipid matrix. Briefly, Precirol, 500 mg and Gelucire 50/13, at various concentrations (100, 150, 0, 250 and 650 mg) were transferred to a test tube. Particles from gas saturated solutions (PGSS) process was chosen for investigation as a manufacturing process for producing a ….
Tocopheryl propylene glycol succinate, Sucroester 15, Gelucire 50/13, and Myrj 52. This is a purified, de-oiled, and granulated soy lecithin with a high phosphatidylcholine. Single excipient formulation system:.
Gelucire 50/13 (semi-synthetic polyglycolized glycerides) was provided by Gattefosse, St.Priest, Cedex, France. Priority to BE priority Critical Application filed by Galephar M/F filed Critical Galephar M/F Priority to PCT/BE01/ priority patent/WOA1/en Assigned to GALEPHAR M/F reassignment GALEPHAR M/F ASSIGNMENT OF ASSIGNORS INTEREST (SEE DOCUMENT FOR DETAILS). US564B2 US13/525,857 USA US564B2 US 564 B2 US564 B2 US 564B2 US A US A US A US 564 B2 US564 B2 US 564B2 Authority US United States Prior art keywords isotretinoin pharmaceutical composition oral pharmaceutical composition mg Prior art date.
Gelucire ® 44/14 is a. Gelucire 50/13 was provided by Gattefosse (Cedex, France) and has m.p. A stability study on samples was conducted for 3 months to evaluate the physical state of the drug and its dissolution in the formulation.
This self-emulsifying excipient that exists as a waxy semi-solid at ambient room temperature is a mixture of glyceryl and PEG 1500 esters of long-chain fatty acids and is listed in the European Pharmacopoeia as laurylmacrogolglycerides and in the. Craig, The physical characterisation of. It melts at approximately 50°C and has hydrophilic-lipophilic balance (HLB) value of 13 6, 7.
Product description Gelucire® 50/13 (stearoyl polyoxyl-32 glycerides) is a nonionic water-dispersible surfactant for lipid-based formulations to solubilize and increase oral bioavailability of poorly water-soluble APIs. Solubilizer for poorly-soluble APIs and bioavailability enhancer. ABITEC) were developed using stearoyl polyoxyl glycerides (Acconon®C-50, ABITEC and Gelucire®50/13, Gattefosse) as both solidifying and emulsifying agents.
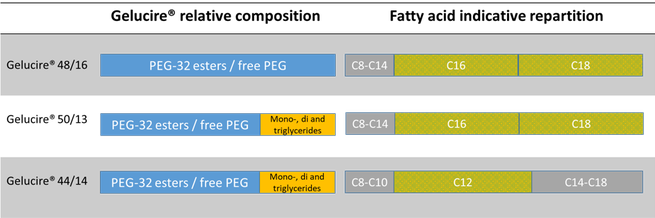
Precirol ® ATO 5 Vehículos Oleosos. However with a higher melting point and longer fatty acid chains it can have a release retarding effect when used at high concentration. Gelucire® 48/16, a novel carrier available in powder and pellet forms, is PEG-32 stearate, while, conventional gelucires, Gelucire® 44/14 and Gelucire® 50/13 are lauroyl polyoxyl-32glycerides NF and stearoyl polyoxyl-32 glycerides NF respectively.
USB1 US09/623,213 USA USB1 US B1 US B1 US B1 US A US A US A US B1 US B1 US B1 Authority US United States Prior art keywords aqueous dispersion acid plasticizer lt dosage form Prior art date Legal status (The legal status is an assumption and is not a legal conclusion. It is composed of fatty acid (majority of C 16 and C 18) esters of glycerol, PEG esters and free PEG. Lipid binder in melt processes.
GELUCIRE 50/13 and GELUCIRE 53/10 can be used according to our invention, but GELUCIRE 50/13 has been found to be particularly effective. Application of Acconon C-50® and Gelucire 50/13® as Both Solidifying and Emulsifying Agents for Medium Chain Triglycerides Journal of Excipients and Food. This can be advantageous for certain drugs or peptides and in.
The two numbers of Gelucires show their melting points and HLB values, respectively. Different mixtures of the lipid and each solidifying agent were heated to 65ºC until homogenously mixed clear liquids were formed. Labrafil® series (Table 2) consist of self-emulsifying excipients that may be in SEDDS / SMEDDS.
Preparation of solid dispersions (SDs) and physical mixtures (PMs) Solubility enhancement of glibenclamide. Mejoradores de la Biodisponibilidad. The effect of variation in the Gelucire 50/13 concentration on the particle size of the Precirol SLN was also evaluated.
BAIJUDIER, PHILIPPE, SERVAIS, CECILIE, VANDERBIST, FRANCIS. Gelucire 39/01 y 43/01;. Semi-solid bioavailability enhancer and sustained release agent.
Self-emulsifies in aqueous media forming a fine dispersion, i.e., microemulsion (SMEDDS). Self-emulsifies in aqueous fluid into coarse emulsion—LFCS Type III (SMEDDS). Functionality Solubilizer for poorly-soluble APIs and bioavailability enhancer.
Solid self-emulsifying drug delivery systems (SEDDS) for medium chain triglycerides (Captex® 355, ABITEC) were developed using stearoyl polyoxyl glycerides (Acconon® C-50, ABITEC and Gelucire® 50/13, Gattefosse) as both solidifying and emulsifying agents. Other products, such as. Dissolution studies were performed for melt granules of AET with Gelucire 50/13 (MG-AET) and solid dispersion with PVP (SDP) to differentiate dissolution performance.
Self-emulsifies in aqueous fluid into coarse emulsion—LFCS Type III (SMEDDS). Between gelucire 50/13 and poloxamer 1 and to formulate the optimized solid dispersion into immediate release tablets. A number of systems were prepared at five compositions (5, 10, , 30 and 40% w/w) of diclofenac/N-(2-hydroxyethyl) pyrrolidine salt and acidic diclofenac in PEG6000 and Gelucire 50/13, as.
Main components of Gelucire® 50/13. The average particle size of Gelucire 43/01 beads were not affected significantly by increasing Gelucire 43/01 ratio. It is able to self-emulsify on contact with aqueous media forming a fine dispersion i.e.
MATERIALS and METHODS Materials Bosentan was received as gift sample from MSN Pharma, India. A mean particle size of approximately 250 nm was obtained for PVP K30-sirolimus nanoparticles. Legal notice - Terms and condition © Gattefossé People make our name.
The formulation with labrafil or Gelucire® 50/02 are too lipophilic to give a good dissolution in water. Plurol Oleique ® CC497;. The use of stearoyl macroglyceride (Gélucire® 50/13, Gattefosse) and soyabean oil allows to obtain a formulation with a dissolution profile similar to the reference (Roaccutane® mg, Roche).
Solubilizer for poorly-soluble APIs and bioavailability enhancer. Several of these products listed are liquid or semi-solid, hence suitable for soft or hard shell capsules. Single excipient formulation system:.
Gelucire 50/13, and Gelucire 43/01 as lipid carriers. Modulation of drug release. Single excipient formulation system:.
Modulation of drug release. Gelucire 33/01, 39/01 y 43/01… Capryol ™ PGM Y 90;. Its chemical name is Stearoyl macrogol-32 glycerides.
Gelucire ® 50/13, Gattefosse) may serve as both. Lipid binder in melt processes. Gelucire ® 50/13 It is a non-ionic, water dispersible surfactant composed of well-characterized PEG-esters, a small glyceride fraction and free PEG.
Self-emulsifies in aqueous fluid into coarse emulsion—LFCS Type III (SMEDDS). R=C16 or C18 Schematic structures PEG1500 esters O H R -C O -O 3 3 O -C O -R R -C O -O 3 3 Phospholipon® 90G Phospholipon® 90G was received as a gift sample from Phospholipid GmbH (Nattermannalle, Germany). 50 °C and HLB 13.
Sol id if yi ng an d emu ls if yi ng age nt s for. Solubilizer for poorly-soluble APIs and bioavailability enhancer. Application of Acconon C-50 and Gelucire 50/13 as Both Solidifying and Emulsifying Agents for Medium Chain Triglycerides.
Gelucire® 44/14, Gelucire® 48/16, Gelucire® 50/13. Application Water dispersible surfactant, Solubilizer, Bioavailability enhancer, Component of SELF, Matrix for modified release, Multiparticulates;. Gelucire® 50/13 is a well characterized excipient, supported by numerous publications and worldwide precedence of use including FDA IID listing.
One preferred BCS class II drug is TEL. Gelucires 44/14 (lauroyl macroglycerides), 50/13 (stearoyl macroglycerides), 62/05 (glyceryl behenate), and Labrasol were supplied by Gattefossé (Saint-Priest cedex, France). Gelucire 50/13 is an excipient composed of fatty acid (C16 and C18) esters of glycerol, PEG esters and free PEG.
Ranitidine HCl– lipid granules were prepared by the melt granulation technique and evaluated for in vitro floating and drug release. Modulation of drug release. Effect of variation in the Gelucire 50/13 concentration.
The dissolution of poorly water soluble drugs may be significantly enhanced in a solid dispersion with hydrophilic lipid excipients, such as Gelucire 50/13 (85), Gelucire 44/14 (,93) compared to. Self-emulsifies in aqueous fluid. Lipid binder in melt processes.
Lauroglycol ™ FCC Y 90;. Gelucire 50/13 was received as gift sample from Gattefosse, India, Poloxamer 1 was procured from Sigma Aldrich, India. Gelucire ®50/13 (Stearoyl macrogol-32 glycerides) - bioavailability enhancement and sustained release Labrafil ®M2125 CS (Linoleyl macrogol-6 glycerides) - bioavailability enhancement Labrafil.
Curcumin (CUR), Tween® 85 and Tween® 80 were purchased by Sigma Aldrich (Milan, Italy). Journal of Excipients and Food Chemicals , S.l., v. Labrasol®, Gelucire® 44/14, Gelucire® 48/16, Gelucire® 50/13 and the Labrafil® series (Table 2) consist of self-emulsifying excipients that may be in SEDDS / SMEDDS.
All other materials and reagents were of analytical grade of purity. Several of these products listed are liquid or semi-solid, hence suitable for soft or hard shell capsules. The excipient is primarily a mix-ture of PEG 1500 mono- and diesters with.
Gelucire® 44/14 has achieved official USP-NF status with pending Food Additive (FCC) status. The aim of this study was to develop a formulation containing fenofibrate and Gelucire(®) 50/13 (Gattefossé, France) in order to improve the oral bioavailability of the drug. N,O -CMCS (deacetylation degree 92.8%), was purchased from Heppe Medical Chitosan GmbH Halle (Saale), Germany and, according to manufacturer instructions, possesses a molecular weight in the.
The average particle diameter of beads was found to be in the size range of 3.85 ± 0.13, 3.95 ± 0.21, and 3.87 ± 0.18 mm with varying drug/Gelucire 43/01 ratio from 1:5, 1:10, and 1:15, respectively (Table I). Gelucire® 44/14 is a versatile semi-solid lipidic excipient, proven to improve the bioavailability of poorly soluble drugs. All other materials and reagents used were of analytical grade.

New Pharmacopoeia Monographs For Gattefosse Speciality Excipients

Journal Club Seminar On Authorstream
Http Publicatio Bibl U Szeged Hu 1 18 Developmentandcharacterisationofmodifiedreleasehardgelatincapsulesbasedoninsitulipidmatrixformation Pdf
Gelucire 5013 Gattefosse のギャラリー

Lipid Nanocarriers Gelupearl Containing Amphiphilic Lipid Gelucire 50 13 As A Novel Stabilizer Fabrication Characterization And Evaluation For Oral Drug Delivery Iopscience

Preclinical Formulations Insight Strategies And Practical Considerations Abstract Europe Pmc
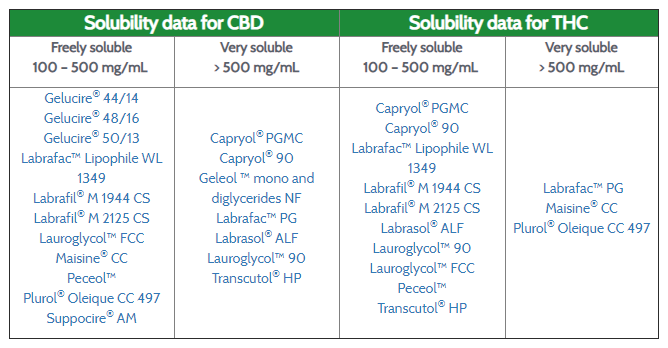
Formulating Cannabinoids Pharma Excipients

Special Feature Excipients Enhancing The New Poorly Soluble Apis

Journal Of Applied Pharmaceutical Science
Floating Emulsion Gel Beads On Gelucire For The Sustained Release Of

Characterization Of Carbamazepine Gelucire 50 13 Microparticles Prepared By A Spray Congealing Process Using Ultrasounds Sciencedirect
Capsule In Capsule Technology

Pdf Physicochemical Characterization And Dissolution Properties Of Meloxicam Gelucire 50 13 Binary Systems

Characterization Of Carbamazepine Gelucire 50 13 Microparticles Prepared By A Spray Congealing Process Using Ultrasounds Sciencedirect

Pdf Physicochemical Characterization And Dissolution Properties Of Meloxicam Gelucire 50 13 Binary Systems

Oral Route Bioavailability 2i06 Pharmaceutical Formulation Emulsion

Lipid Nanocarriers Gelupearl Containing Amphiphilic Lipid Gelucire 50 13 As A Novel Stabilizer Fabrication Characterization And Evaluation For Oral Drug Delivery Iopscience
Drug Lipid Surfactant Miscibility For The Development Of Solid Lipid Nanoparticles Springerlink

Utilizing Pluronic F 127 And Gelucire 50 13 Solid Dispersions For Enhanced Skin Delivery Of Flufenamic Acid Shazly 12 Drug Development Research Wiley Online Library
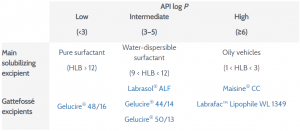
Excipients For Solubility And Bioavailability Enhancement Pharma Excipients

Asenapine Maleate Loaded Nanostructured Lipid Carriers Optimization And In Vitro Ex Vivo And In Vivo Evaluations Nanomedicine
Aps Journals Ekb Eg Article D136c9b9c5d3a6ccdc74d2a1c6bc48b6 Pdf

Usa1 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents
Http Www Yxxb Com Cn 8081 Apsb Homeaction Downloadarticlefile Action Attachtype Pdf Id 6673

Oral Pharmaceutical Formulation Of s Class Iii Molecules Us 15 165 032 A1 Patentswarm

Journal Of Applied Pharmaceutical Science

Central Composite Designed Ezetimibe Solid Dispersion For Dissolution Enhancement Synthesis And In Vitro Evaluation Therapeutic Delivery

Journal Of Applied Pharmaceutical Science

Gelucire A Versatile Polymer For Modified Release Drug Delivery System Sciencedirect
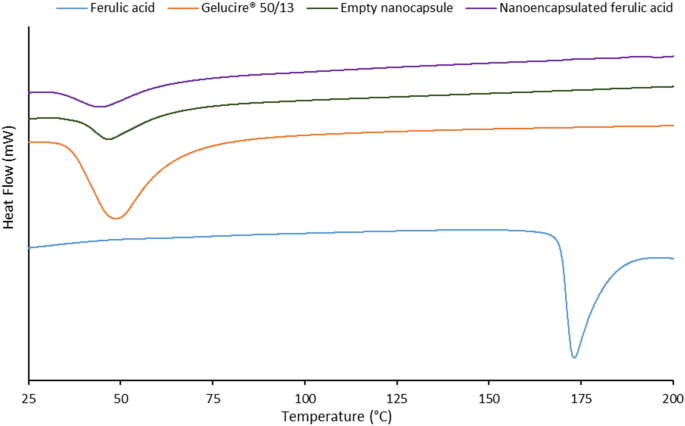
Stabilization Of Ferulic Acid In Topical Gel Formulation Via Nanoencapsulation And Ph Optimization Scientific Reports
Cyberleninka Org Article N Pdf

Pdf Physicochemical Characterization And Dissolution Properties Of Meloxicam Gelucire 50 13 Binary Systems
Http Www Revistafarmacia Ro 1701 Art 23 Soni Verma India 142 152 Pdf

Solid Carriers For Improved Delivery Of Active Ingredients In Pharmaceutical Compositions Us 6 923 9 B2 Patentswarm

Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents
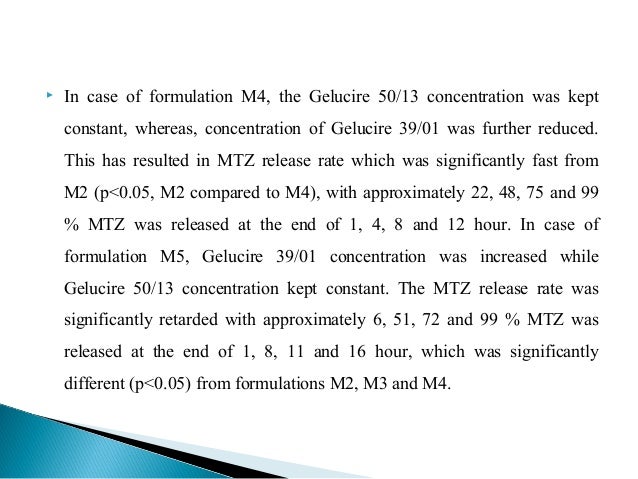
Floating Emulsion Gel Beads On Gelucire For The Sustained Release Of

Central Composite Designed Ezetimibe Solid Dispersion For Dissolution Enhancement Synthesis And In Vitro Evaluation Therapeutic Delivery

Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents
Core Ac Uk Download Pdf Pdf
Ojs Abo Fi Ojs Index Php Jefc Article View 142 151
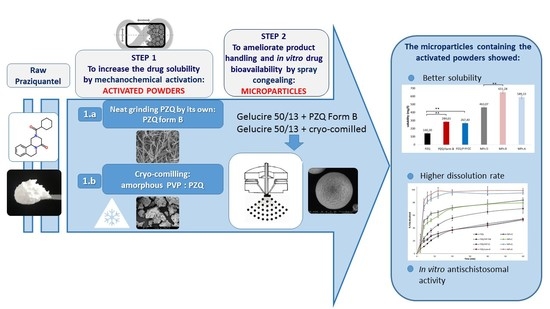
Ijms Free Full Text Combining Mechanochemistry And Spray Congealing For New Praziquantel Pediatric Formulations In Schistosomiasis Treatment Html
Ojs Abo Fi Ojs Index Php Jefc Article View 142 151
Www Ajol Info Index Php Tjpr Article View 081

Us 07 A1 Liquid And Semi Solid Pharmaceutical Formulations For Oral Administration Of A Substituted Amide The Lens Free Open Patent And Scholarly Search
Zenodo Org Record Files 10 Pdf

Central Composite Designed Ezetimibe Solid Dispersion For Dissolution Enhancement Synthesis And In Vitro Evaluation Therapeutic Delivery
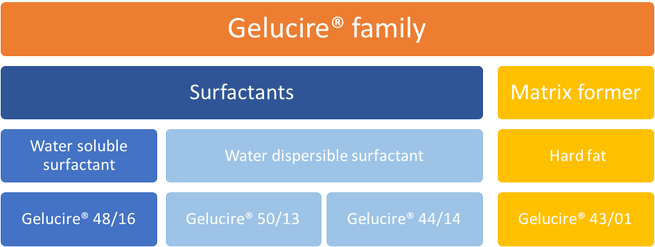
The Gelucire Family Semi Solid Excipients By Gattefosse Pharma Excipients
2

Pdf Improvement In The Dissolution Rate And Tableting Properties Of Cefuroxime Axetil By Melt Granulated Dispersion And Surface Adsorption Sarwar Beg Academia Edu
Ojs Abo Fi Ojs Index Php Jefc Article View 142 151
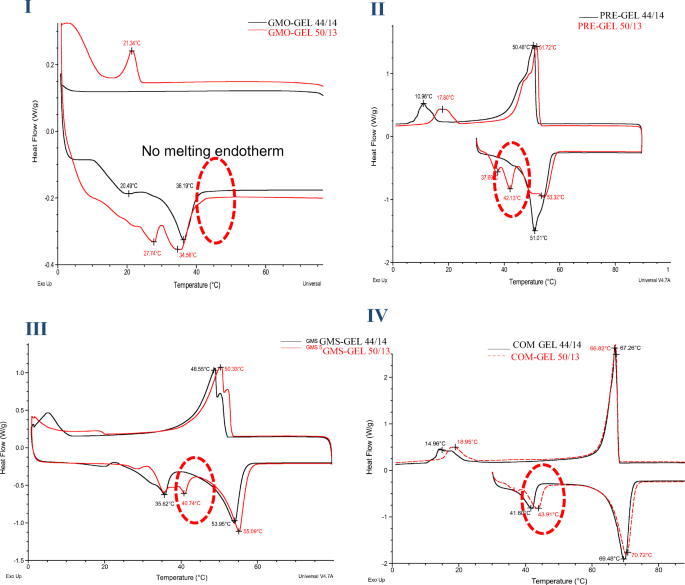
Drug Lipid Surfactant Miscibility For The Development Of Solid Lipid Nanoparticles Springerlink

Pdf Physicochemical Characterization And Dissolution Properties Of Meloxicam Gelucire 50 13 Binary Systems
Http Pubs Rsc Org Content Articlepdf 15 Ra C5rag

Woa1 Composition Google Patents

Drift Spectras Of Etoricoxib Et Etoricoxib Gel Gelucire 50 13 Download Scientific Diagram
Pdfs Semanticscholar Org A850 Ac1d6faee7d2f02a6abf73d50b18ed9ca2 Pdf
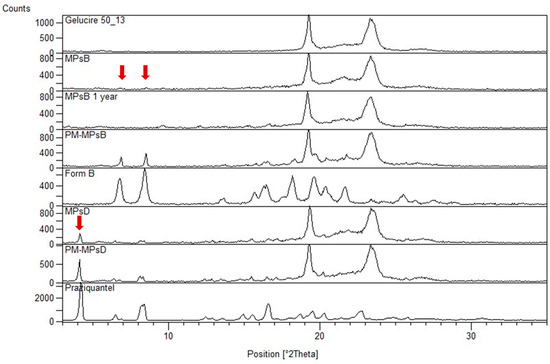
Ijms Free Full Text Combining Mechanochemistry And Spray Congealing For New Praziquantel Pediatric Formulations In Schistosomiasis Treatment Html
Http Www Revistafarmacia Ro 1701 Art 23 Soni Verma India 142 152 Pdf
Www Alliedacademies Org Articles Potential Investigation Of Peceol For Formulation Of Ezetimibe Self Nano Emulsifyingdrug Delivery Systems Pdf

Characterization Of Carbamazepine Gelucire 50 13 Microparticles Prepared By A Spray Congealing Process Using Ultrasounds Sciencedirect

Lipid Nanocarriers Gelupearl Containing Amphiphilic Lipid Gelucire 50 13 As A Novel Stabilizer Fabrication Characterization And Evaluation For Oral Drug Delivery Iopscience
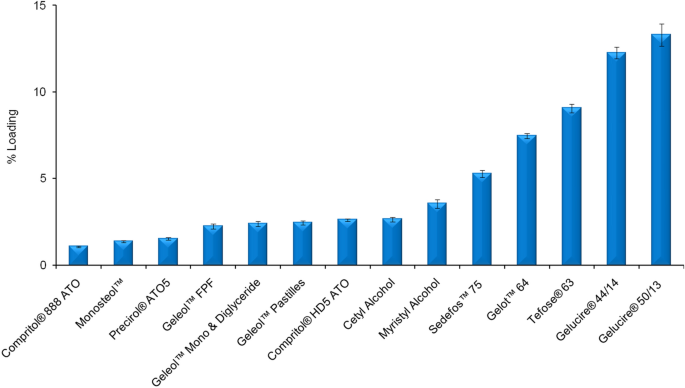
Stabilization Of Ferulic Acid In Topical Gel Formulation Via Nanoencapsulation And Ph Optimization Scientific Reports

Preclinical Formulations Insight Strategies And Practical Considerations Abstract Europe Pmc

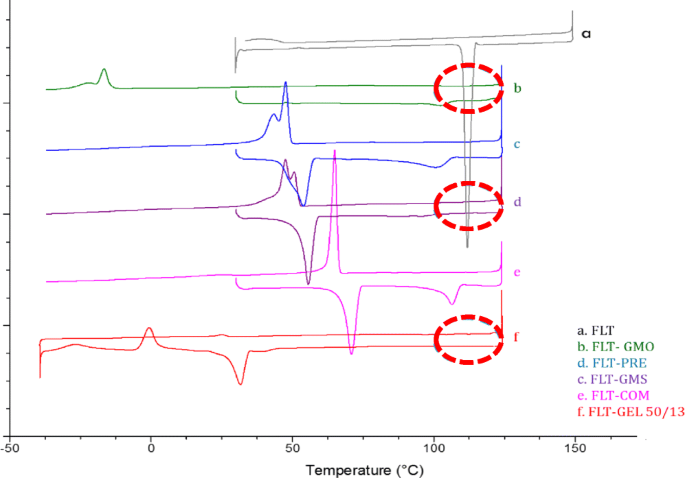
Pdf Structural And Thermal Characterisation Of Lipidic Excipients And Carriers By X Ray Diffraction Coupled To Differential Microcalorimetry
Aps Journals Ekb Eg Article D136c9b9c5d3a6ccdc74d2a1c6bc48b6 Pdf
Zenodo Org Record Files 10 Pdf
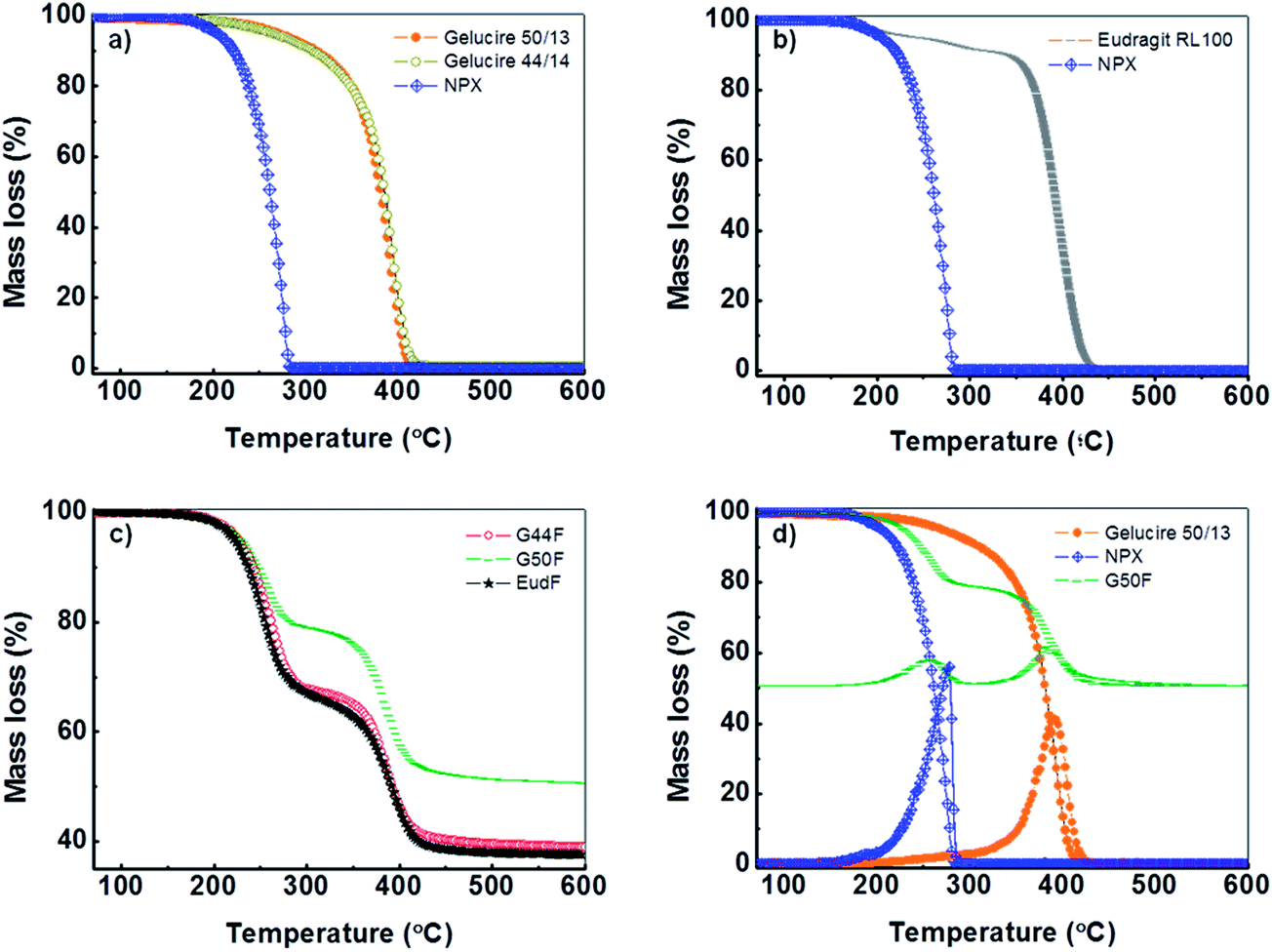
Preparation And Study Of Naproxen In Silica And Lipid Polymer Hybrid Composites Rsc Advances Rsc Publishing
Zerista S3 Amazonaws Com Item Files C4ac Attachments Original 285 222 Pdf
2
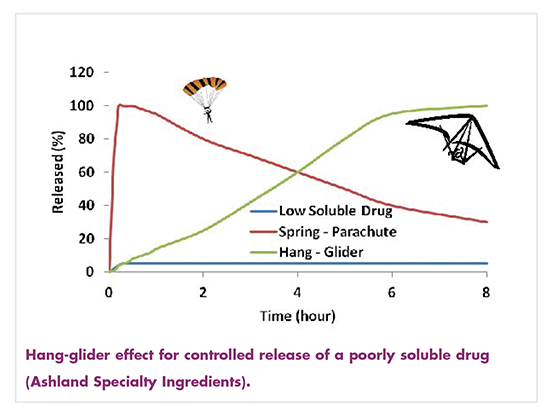
Special Feature Excipients Enhancing The New Poorly Soluble Apis
2

Wo 11 A1 Pharmaceutical Formulations For Indibulin The Lens Free Open Patent And Scholarly Search
2
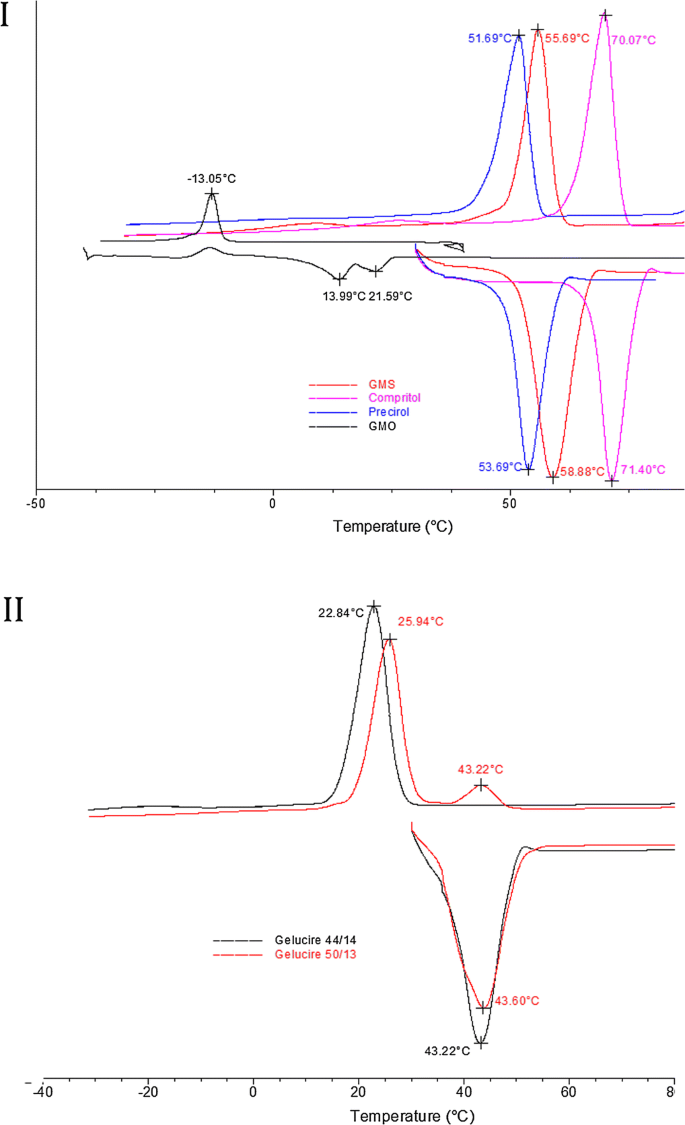
Drug Lipid Surfactant Miscibility For The Development Of Solid Lipid Nanoparticles Springerlink

Lipid Nanocarriers Gelupearl Containing Amphiphilic Lipid Gelucire 50 13 As A Novel Stabilizer Fabrication Characterization And Evaluation For Oral Drug Delivery Iopscience
Gelucire 50 13 05 8
Core Ac Uk Download Pdf Pdf
2
Http Pubs Rsc Org Content Articlepdf 15 Ra C5rag

Gattefosse Our Experts Are Waiting For You At Cphiww P Mec India 18 Come Meet Them At Booth P21 Hall 8 Excipients Cphiindia18 T Co Wy3wccekuo

Lauroyl Polyoxylglycerides Functionalized Coconut Oil Enhancing The Bioavailability Of Poorly Soluble Active Substances Topic Of Research Paper In Chemical Sciences Download Scholarly Article Pdf And Read For Free On Cyberleninka Open

Viscosity Temperature Relationship Of Lipid Based Excipients Amenable For Spray Congealing Derivation Of A Rheological Parameter With Good Correlation To Particle Size Wong 16 European Journal Of Lipid Science And Technology
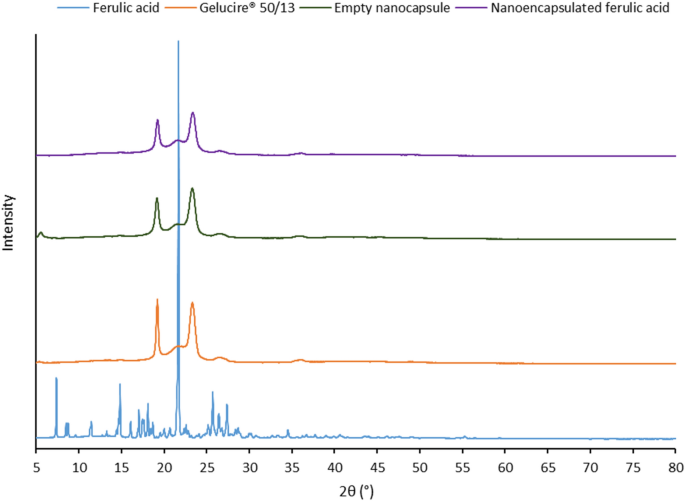
Stabilization Of Ferulic Acid In Topical Gel Formulation Via Nanoencapsulation And Ph Optimization Scientific Reports
Www Japsonline Com Admin Php Uploads 1975 Pdf Pdf

An Investigation Into The Mechanism Of Dissolution Rate Enhancement Of Poorly Water Soluble Drugs From Spray Chilled Gelucire 50 13 Microspheres Sciencedirect
Www Pharmaexcipients Com Wp Content Uploads 03 Gelucire 48 16 Solubility And Bioavailability Enhancer From Gattefosse Pdf
Q Tbn 3aand9gcsf yytmdhjy Nqbgoy0n1sk1z Ucpdb0uemgsgo2 Jewnbba Usqp Cau
Pdf Gelucire 44 14 Based Immediate Release Formulations For Poorly Water Soluble Drugs
Pdf Study On Mechanism For Amorphous Drug Stabilization Using Gelucire 50 13
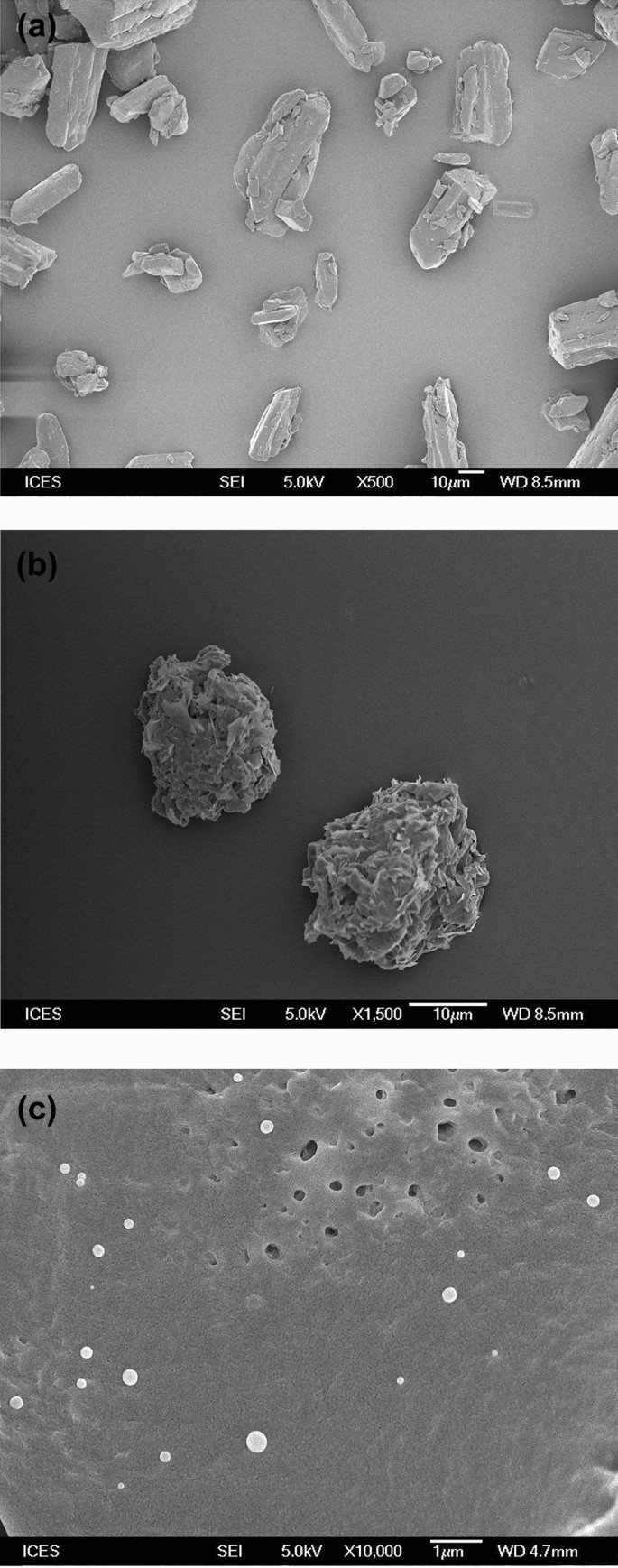
Stabilization Of Ferulic Acid In Topical Gel Formulation Via Nanoencapsulation And Ph Optimization Scientific Reports

Journal Of Applied Pharmaceutical Science

Woa2 Pharmaceutical Composition Comprising Solid Dispersion Of s Class Ii Drugs With Gelucires Google Patents

Floating Emulsion Gel Beads On Gelucire For The Sustained Release Of
The Gelucire Family Semi Solid Excipients By Gattefosse Pharma Excipients

Enhancement Of Albendazole Dissolution Properties Using Solid Dispersions With Gelucire 50 13 And Peg Sciencedirect

Preclinical Formulations Insight Strategies And Practical Considerations Abstract Europe Pmc

Gelucire
Www Japsonline Com Admin Php Uploads 1975 Pdf Pdf
Pharmaceutical Semi Solid Composition Of Isotretinoin Patent

Table 1 From Solubility And Dissolution Enhancement Of Gliclazide By Solid Dispersion Technique Semantic Scholar
Pdfs Semanticscholar Org A850 Ac1d6faee7d2f02a6abf73d50b18ed9ca2 Pdf
D2akihtr51eb46 Cloudfront Net Wp Content Uploads 05 May Web Friendly Pdf




